Study Design
CKiD is a prospective, observational cohort of children, adolescents, and young adults with chronic kidney disease. The exposures and outcome of interest are measured at a participant’s first study visit, and then continually collected at annual follow-up visits, allowing investigators to understand how CKD and the health of this population change over time.
There are many outcomes of interest measured at CKiD yearly follow-up visits. A few of them include measures of kidney function such as GFR and urine protein to creatinine ratio, neurocognitive function, markers of risk factors for cardiovascular disease, growth, and other co-morbid conditions. CKiD leverages the power of the cohort study design through these regularly scheduled visits where markers of disease progression can be measured in a standardized setting. CKiD also collects data on important clinical events that are particularly meaningful to this population, specifically the onset of end-stage kidney disease (ESKD) and death.
CKiD also follows participants after transplant and initiation of dialysis (kidney replacement therapy (KRT)). Collecting data on participants in this phase allows investigators to understand the determinants of long-term positive outcomes post-KRT.
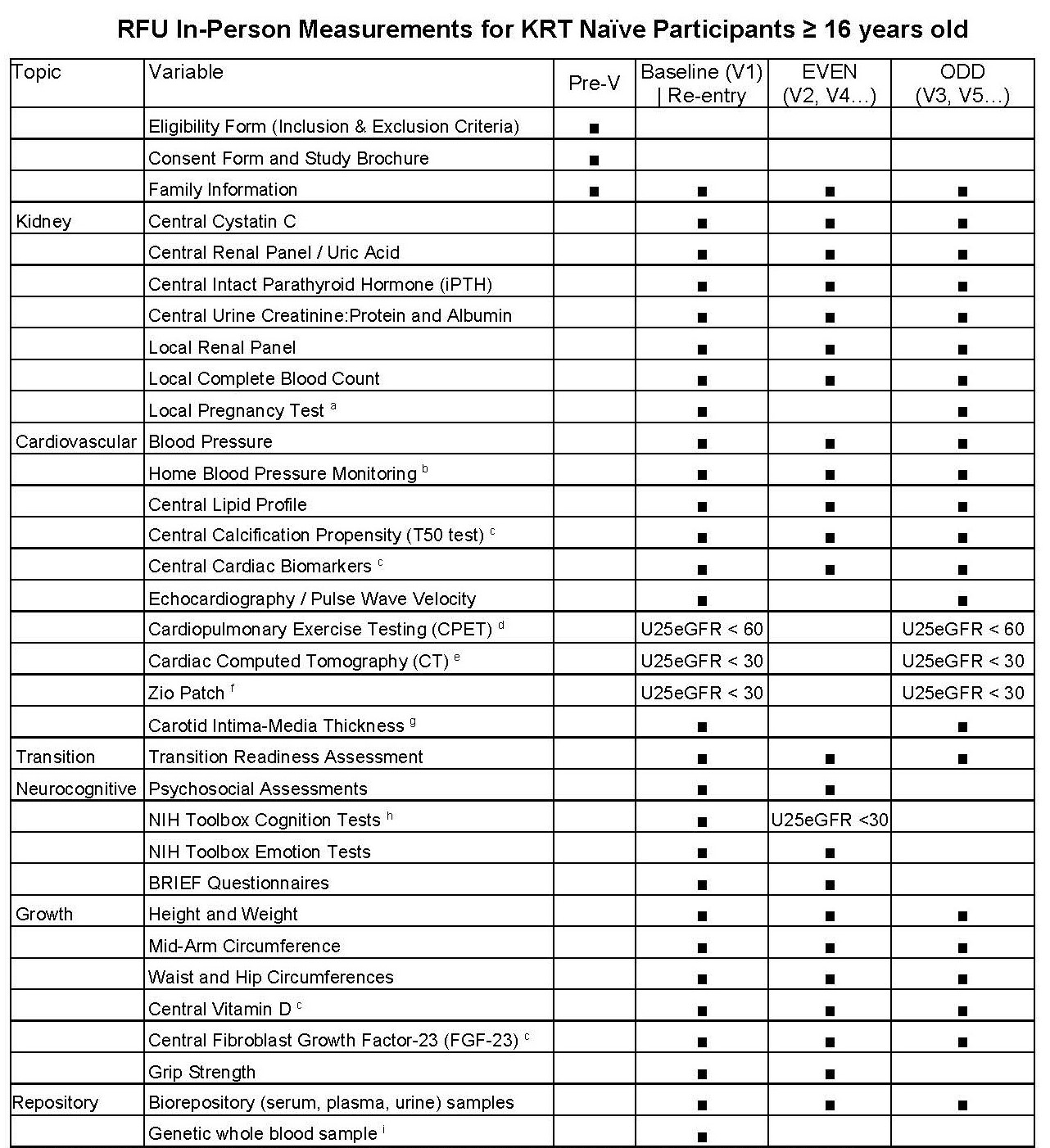
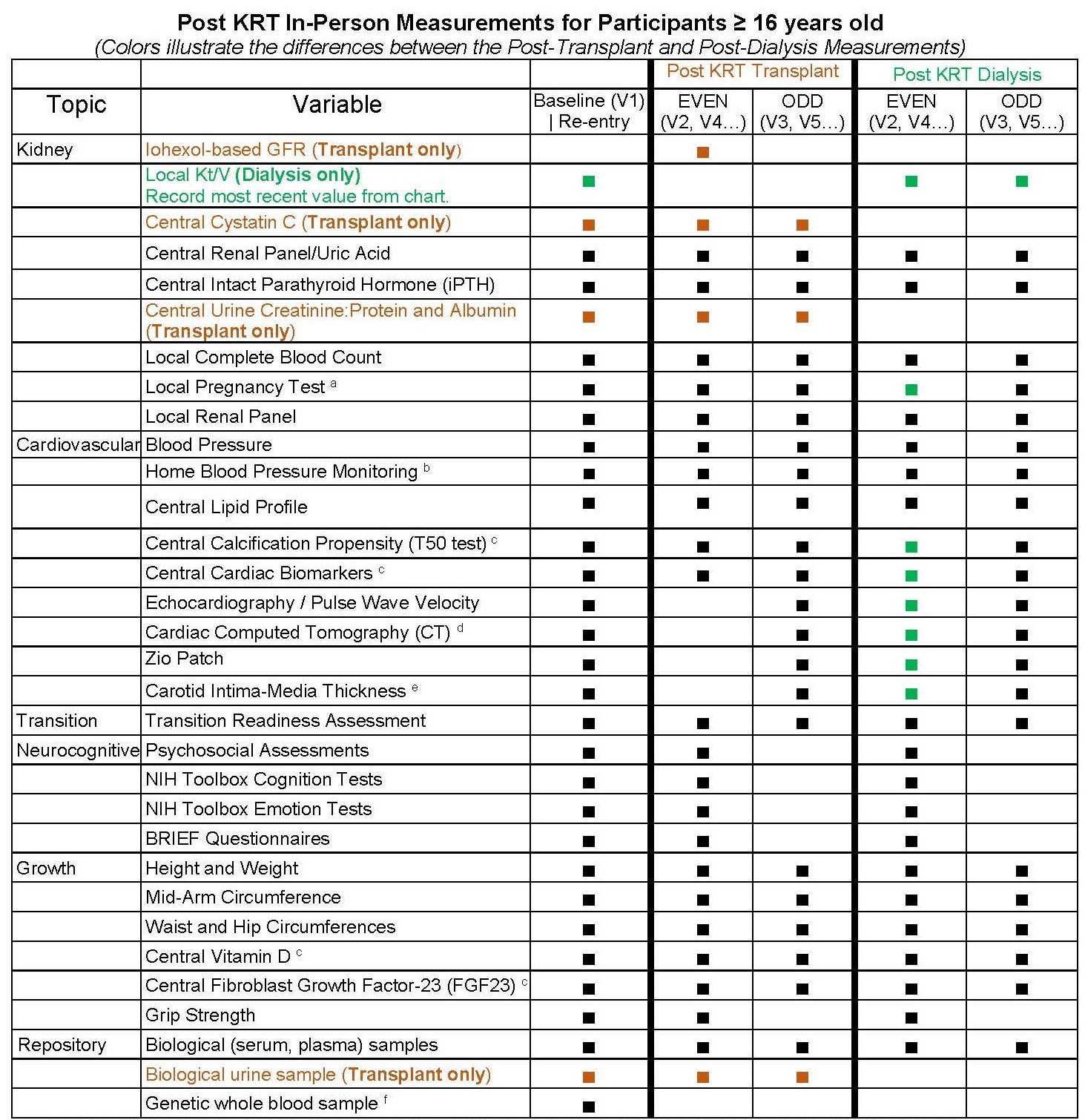
For a full description and schedule of CKiD study visits, please see the schematics below.
Study Population
The CKiD Study enrolls children and adolescents with mild-to-moderate CKD of both glomerular and non-glomerular disease etiology. CKiD has been enrolled in 3 waves: Cohort 1, Cohort 2, Cohort 3, and currently enrolling Cohort 4.
Cohort 1 enrolled approximately 600 racially and ethnically diverse children between the ages of 1 and 16 with GFRs between 30 and 90 ml/min|1.73m2. Cohort 2 enrolled approximately 300 children with slightly more mild CKD compared to those in Cohort 1, as the GFR requirement was between 45 and 90 ml/min|1.73m2. Cohort 3 enrolled roughly 200 participants between the ages of 6 months and 16 years with a non-glomerular CKD diagnosis within 5 years of being enrolled. Finally, Cohort 4, the most recent cohort, is enrolling participants 16 to 22 years old with moderately impaired kidney function defined as GFR <60ml/min|1.73m2, and those who have initiated kidney replacement therapy (dialysis or transplantation).
Please see below for specific details on the inclusion and exclusion criteria of the CKiD Study.
Inclusion & Exclusion Criteria
Inclusion Criteria
General Inclusion Criteria
- Age between 1 and 16 years (before 17th birthday) for Cohorts 1 and 2; age between 6 months and 16 years (before 17th birthday) for Cohort 3; age between 16 to 22 years (before 23rd birthday) for Cohort 4 and regularly seen by pediatric nephrologist prior to enrollment
- Estimated (based on SCr) Schwartz GFR between 30 and 90 ml/min|1.73m2 for Cohort 1 OR an estimated GFR between 45 and 90 ml/min|1.73m2 based on the updated Schwartz formula for Cohort 2; an estimated GFR ≤60 based on the CKiD Under 25 estimating equation (U25eGFR) OR KRT experience (dialysis or transplant) for Cohort 4
- Willingness and ability to provide informed consent and assent
- For Cohort 2, an equal distribution of children with glomerular and non-glomerular causes of disease were enrolled (i.e., 150 within each) and the study placed an upper limit of 60% for the percent of enrolled with non-glomerular disease
- For Cohort 3, 190 children with non-glomerular diagnosis and duration of kidney disease less than 5 years were enrolled
- For Cohort 4, 100 adolescents and young adults (AYA) with moderately impaired kidney function defined as U25eGFR <60ml/min|1.73m2 (KRT-naïve) and 100 AYAs who have initiated kidney replacement therapy (KRT experience) will be enrolled
Exclusion Criteria
The following conditions comprise the exclusion criteria for Cohort 4:
- Solid organ (other than kidney), bone marrow or stem cell transplantation
- Cancer diagnosis and receiving treatment or within 12 months post completion of treatment
- HIV diagnosis/treatment and receiving treatment or within 12 months post completion of treatment
- Current pregnancy or pregnancy within past twelve months
- Inability to complete major data collection procedures
- Not fluent in English or Spanish
- Plans to move out of area of any participating CKiD site (Families can be transferred to another CKiD site if they move)
- Existing moderate to severe congenital structural heart disease
- Genetic syndromes involving the central nervous system (e.g., Down syndrome)
- History of Severe to Profound Intellectual Disability (i.e., IQ < 40, significant impairment in adaptive functioning and/or inability to independently execute self-care skills)
- For cohorts 3, children who were expected to receive kidney replacement therapy within 6 months of date of enrollment were not recruited
Study Visit Schedule for Regular Follow-Up
Study Visit Schedule for Post-KRT Follow-Up
Click Here to download a combined*, PDF version of the Regular Follow-up (RFU) and Post-KRT CKiD Visit Schedules for participants ≥ 16 years and older.
*Document also includes Abbreviated RFU and Post KRT Visit Schedule for participants who are < 16 years old. [/av_textblock] [/av_one_full]